The development of smokeless powder in the late 19th century marked a radical change in gun propellants. Previously, all powder had been a mechanical mixture of oxidizers and fuels, and had turned less than 50% of its weight into gas, with the rest (mostly potassium compounds) making up the bulk of the smoke. Smokeless powders were single substances which could turn completely into gas, reducing smoke and increasing the energy released.

Paul Vieille
The first chemical explosive to be discovered was nitrocellulose, also known as guncotton, in 1846. Nitrocellulose is produced by the application of concentrated nitric acid on cellulose, the usual source of which is either cotton or wood shavings. Early nitrocellulose was unstable and dangerous to make, and a practical method didn't arrive until the 1860s. Even then, it burned entirely too quickly to be suitable for use in guns, because it was still cotton-like, allowing virtually the entire mass to burn at once. Instead, it was used primarily in torpedoes and mines and for blasting purposes. It wasn't until 1884 when the first proper smokeless powder was produced, by French chemist Paul Vieille. His Poudre B consisted of a mix of different types of nitrocellulose, some with more nitro groups than others, gellatinized using ether and an alcohol. It was then rolled into flat sheets, dried, and cut into strips or squares. This meant that only a relatively small proportion could burn at any one time, spreading out the gas production and making it a viable propellant.1
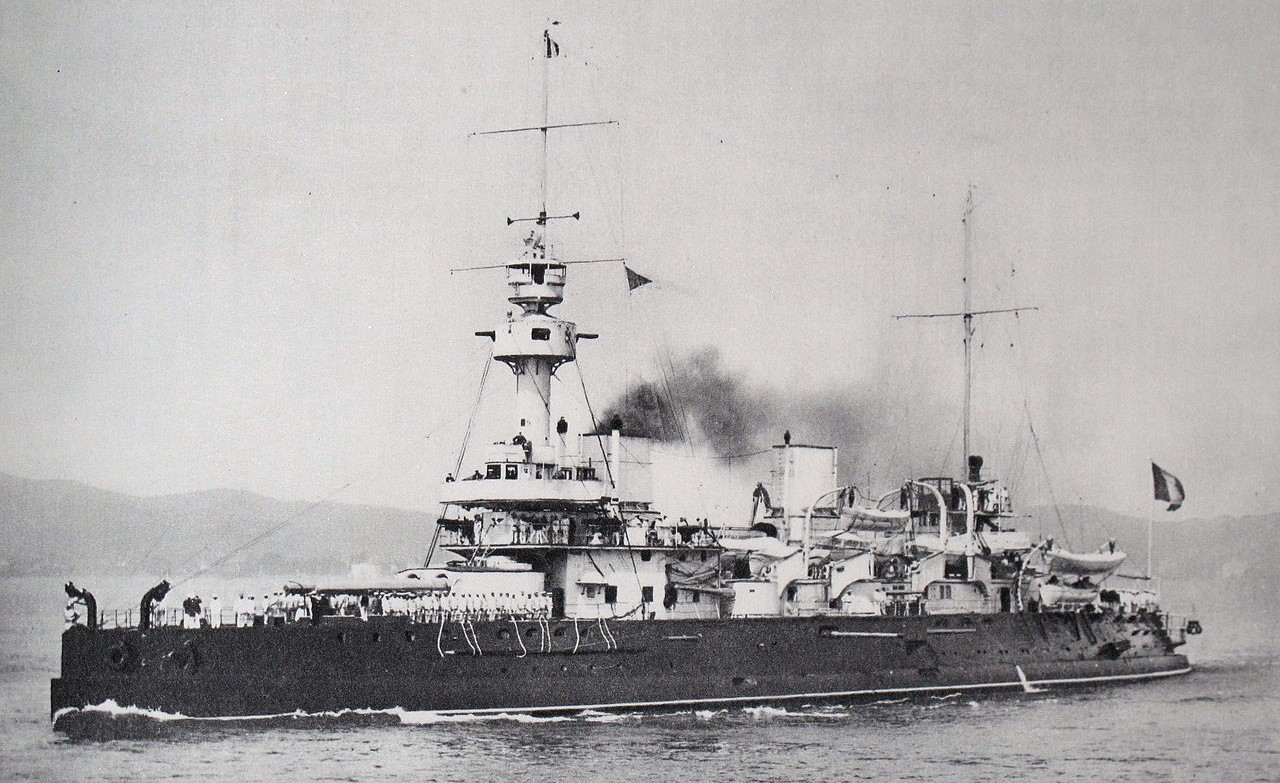
Brennus, which I believe to be the first battleship equipped with smokeless powder
The new powder allowed the French to cut the weight of the charges they used in their existing guns by a third for the same performance, or to gain a significant increase in muzzle velocity without a major boost in pressure, thanks to the powder's slower burn rate. It also made the guns safer in several ways. First, the new powder was harder to ignite than black powder,2 usually requiring a booster charge of black powder to start it burning. Second, the burning rate was sensitive to pressure, so if a pile of it in the open was set on fire, it would burn quite slowly. Third, the powder left less residue in the gun, which allowed the gun crews to avoid sponging out the gun, which had previously been necessary to quench any embers left over from the previous shot. Poudre B was also lightly coated with graphite, which retarded the initial burning somewhat, and made the powder conductive, preventing static electricity from building up and forming a safety hazard.

Alfred Nobel
Poudre B was followed by other smokeless powders, the first of which was ballistite, invented by Alfred Nobel.3 This was a mix of nitroglycerine and nitrocellulose that gave even higher performance than Poudre B. It was the first of the so-called double-base powders, known as such because they use both nitrocellulose and nitroglycerine, while nitrocellulose-only powders are known as single-base powders. The British government set up a committee to develop its own powder, which in 1889 produced a variant of ballistite known as cordite, because it was squeezed out in long cords. It was composed of 37% nitrocellulose, 58% nitroglycerine, and 5% mineral jelly.4 Nobel filed a lawsuit, which after a major legal battle eventually failed on the grounds that cordite used insoluble nitrocellulose5 instead of the soluble type specified in the ballistite patent.

Cordite
The early forms of cordite proved to be tremendously powerful, with some guns seeing a fourfold reduction in charge weight relative to brown powder. However, it burned very hot, which made it tremendously erosive. To solve this, a new formulation, known as Cordite MD, was introduced starting in 1901. This was 65% nitrocellulose, 30% nitroglycerine, and 5% mineral jelly. There was a slight reduction in energy output, with a corresponding increase in required charge weight, but it was worth it to double barrel life. Unfortunately, even though it was chemically superior, cordite was the wrong shape, lacking in most cases6 the internal perforations that give a more consistent rate of gas production when burning. The British apparently believed that this would give the smallest charge weight, although it's unclear why they didn't switch to tubes later on. My best guess would be ease of manufacture and resistance to mechanical damage, as the cordite was unlikely to break in ways that led to significant changes in gas production.

Dimitri Mendeleev
All of the other major powers quickly adopted smokeless powder, too, usually some form of ballistite. The only major powers besides France to buck the double-base trend were the US and Russia. Russia's early powder formulations were developed by Dmitri Mendeleev, famous for inventing the periodic table, although they were still using black powder during the Russo-Japanese war a decade later because of cost. The US developed its powder technology mostly independently, although some of Mendeleev's work provided a starting point. The development work was done at Newport, Rhode Island, but the production plant was built at Indian Head, Maryland. The actual process began by washing and drying cotton linters, then nitrating them with a mix of nitric and sulfuric acid. Once that was done, the nitrocellulose was boiled to wash out the acid, then pulped to remove impurities. After that, the result was placed in alternating bathes of cold and boiling water to purify it further. After a test for stability, it was wrung to get out most of the water, and the rest was replaced with alcohol. Ether was then added, and the result was pressed to produce the powder grains.
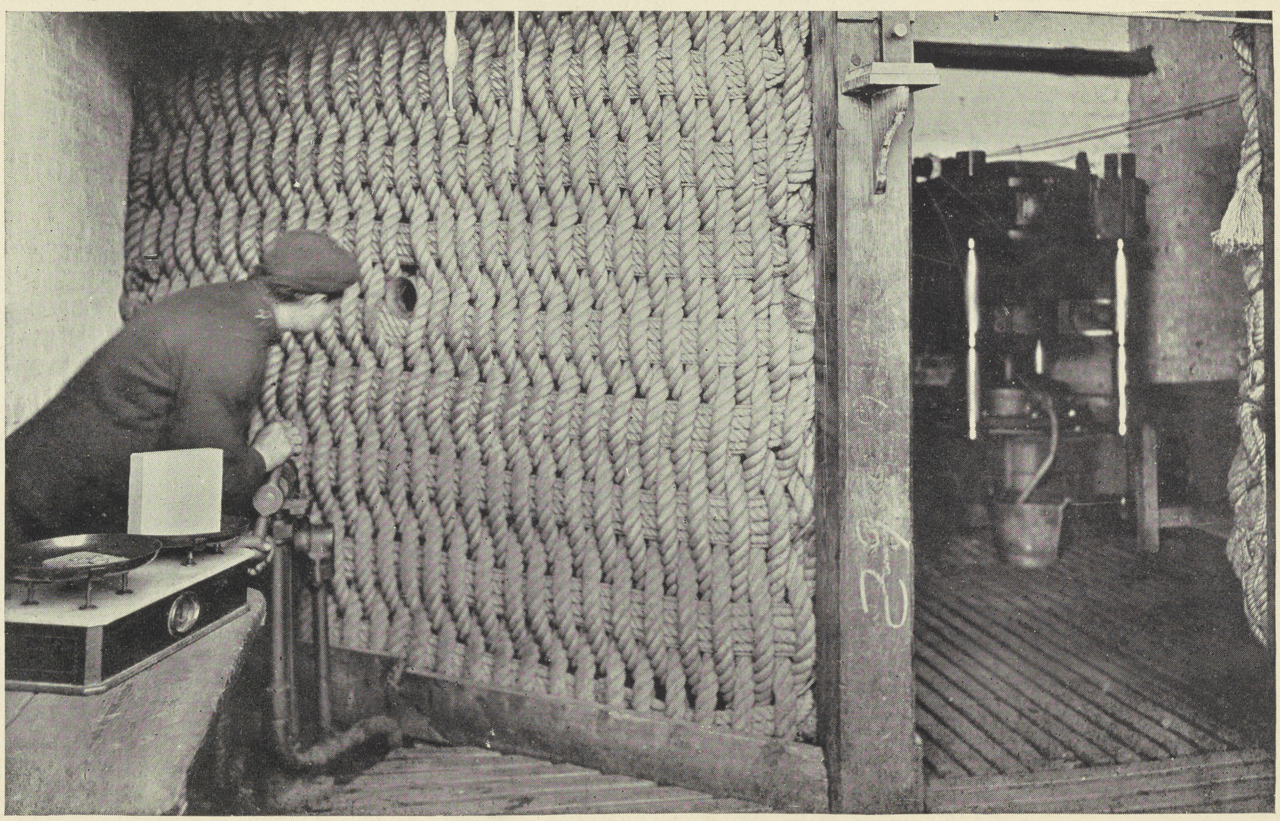
A worker operates a powder press from behind a protective rope screen
As you will notice, most of these steps were concerned with making sure the nitrocellulose was pure, and there was good reason for this. If there were any impurities, particularly any acid left by the manufacturing process, they would begin to decompose the powder. To make matters worse, the resulting decomposition products also catalyzed powder decomposition, so the rate tended to increase, eventually leading to serious instability. This was graphically demonstrated numerous times during the early years of smokeless powder, most prominently in France. In 1907, while in a Toulon drydock the French battleship Iéna suffered a magazine explosion. Efforts to fight the fire were hindered by the fact that she was in drydock and her seawater system was thus inoperative. In particular, the magazines couldn't be flooded, a problem the captain of the nearby battleship Patrie attempted to rectify by shooting at the dock gates. The shell bounced off, and the dock had to be flooded normally.
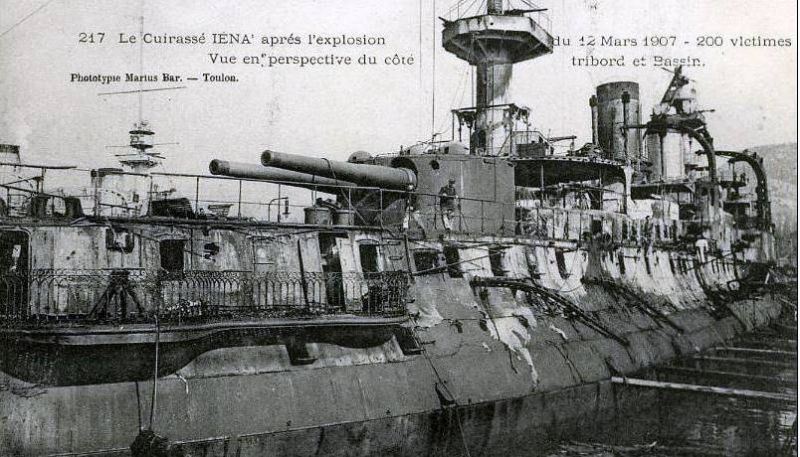
Iena after the explosion
Suspicion for the accident immediately focused on Poudre B, thanks in part to the large clouds of yellow smoke produced by the conflagration.7 The suspicion was strengthened when records were checked and they discovered that two-thirds of the 12" charges and 86% of the 4" charges onboard a year previously were over six years old. Even if the propellant is free of acid, it loses solvents over time, solvents which are important for stability. Keeping the powder in sealed canisters helps with this, although at some point, the only option is to rework the powder by grinding it up and recasting it. The French had not done this, nor had they taken many steps against the other scourge of powder stability, heat. Refrigeration equipment would quickly become standard in the magazines of most warships.
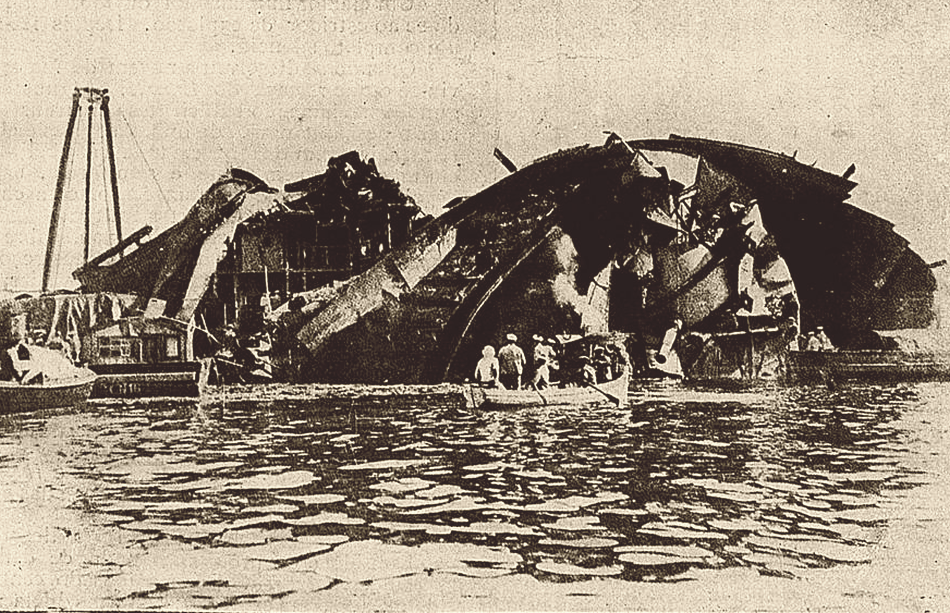
The remains of Liberte
But whatever corrective measures the French took were insufficient to prevent further tragedy four years later. While moored in Toulon, a fire broke out aboard the battleship Liberte, and despite the crew's efforts to fight it, the ship blew up 20 minutes later, killing almost 300 onboard and throwing debris into the surrounding ships, where 60 more men died. The problem was quickly traced to the powder, which was suspected to have overheated, and the magazine-flooding arrangements, which didn't work. The French instituted a rule against carrying any powder more than four years old, and the problems stopped, at least for them.
Nor were the French alone in these problems. Japan suffered peacetime explosions in Mikasa in 1905 and Matsushima in 1908,8 while the Brazilians lost the battleship Aquidabã in 1906. All three of these ships used cordite, and the Japanese at least imported theirs from Britain. The Russian nitrocellulose propellant was no safer, with the battleship Borodino suffering from a magazine explosion at Tsushima.

An American smokeless powder press
The US alone seemed to avoid these problems.9 Some of this was due to the rigorous quality control in the US process, and some was the composition of the powder itself. The use of volatile solvents in the powder made it considerably more stable, although the British considered it rather "wet" and low-powered. American powder additives also helped with safety. The first of these was rosaniline dye, added in 1905. In and of itself, it didn't stabilize the powder, but it did change the powder's color when acid began to form, marking it out for disposal. Three years later, diphenylamine was added, working as a true stabilizer to capture and neutralize any acid breakdown products. It was so effective that lots of powder manufactured in the 1940s were used aboard the Iowa class when they were reactivated 40 years later.
By the time war broke out in 1914, it looked as if most of the kinks in smokeless powder had been worked out. The new powders had been a key factor in the rapid development of naval guns, and they would now be put to the test. We'll pick up the story there next time.
1 Note that the squares, because they were quite thin, burned primarily on the faces, and thus produced gas at a near-constant rate. ⇑
2 Interestingly, this is despite a lower ignition temperature than black powder, because smokeless powder conducts heat much better. ⇑
3 Yes, that Alfred Nobel. ⇑
4 The mineral jelly was intended to serve as a lubricant, but in fact did nothing of the sort. It was a reasonably effective stabilizer, locking up any decomposition products, at least for a little while. ⇑
5 Nitrocellulose can be dissolved in alcohol so long as the nitrogen content is below 12%. ⇑
6 There were a few weapons that used tubular cordite, but they were all smaller guns. ⇑
7 Poudre B tended to produce nitrogen dioxide, which would then turn into yellow nitric acid. ⇑
8 These may have been caused by the practice of brewing alcohol by Japanese enlisted men. ⇑
9 I know that the obvious objection is the explosion aboard the Maine in 1898. Maine didn't have smokeless powder onboard. The first USN ship to get that was the cruiser Marblehead, commissioned in 1897. Note that several very reputable sources, including Friedman and Campbell, assert otherwise, but I've checked specialist articles on the Maine, and they all agree on this. There were also explosions aboard Missouri in 1904 and Kearsage in 1905, although both were set off by flashback from open gun breeches. ⇑

Comments
"Nitrocellulose is produced by the application of nitrogen on cellulose,..."
Should be "...application of concentrated nitric acid to...". This is not 100% correct, but I assume you don't want to get into the weeds of nitration chemistry.
For those that do want to get into the weeds, the reference I have in front of me recommends a mixture of 21% Nitric Acid, 63% Sulfuric Acid and the balance water. 14.5kg cellulose & 682kg acid cook for 25min at 30C. : )
That was a mistake. Should be fixed now. And I didn't want to get deep into the guts of nitration chemistry. There are actually books written on that kind of stuff, and I'm working on the wider technical aspects.
Looking over it again the one thing I feel needs adding is stating that the decomposition is auto-catalytic - acid speeds the decomposition and the decomposition makes more acid. Without knowing that, some of the parts about stabilizers and storage length don't make as much sense.
Excellent work as always, bean.
Also a good point, and fixed.
So the most eyebrow raising thing I learned from following links in the is article was that celluloid is just a plasticized polymer solution of nitrocellulose and camphor, which turns out to be exactly as flammable as one would expect. The idea that so many things could be made out of what was mostly guncotton for so long is amazing to me.
I recently ran across a reference which says that naval shipyards tended to require glass plate film instead of cellulose because of the hazard. This lasted until safety film was developed in the 30s, and appears to have applied in both the US and Britain.
"And I didn’t want to get deep into the guts of nitration chemistry"
One of my favorite facts about nitration chemistry in the late 19th century is the use of one-legged stools in nitroglycerine plants. Nitroglycerine used to be made by nitrating glycerine in ~1tonne cooled reactors, with an operator watching a thermometer. If the temperature rose too high, and a runaway reaction became likely then the operator would dump the whole tank into a pond to quench the reaction. The operators would sit on one-legged stools so they couldn't fall asleep.
Of course, now nitration is done in small (litre scale) continuous reactors, so the total inventory of nitroglycerine in a plant is orders of magnitude smaller, and so is any resulting explosion.
The flammability of celluloid has caused the catastrophic loss of several film archives, and is responsible for film archive buildings being remarkably more fire- and explosion-safe than one might naively expect.
Did Germany also have problems with powder explosions? In this period they were THE world leaders in chemistry / chemical engineering, ahead even of the Americans.
I don't believe Germany ever had any serious problems with powder explosions, no. But I'm also not as read up on their navy as I am on some others.
Yeah, all the best sources on the XIX cent German Navy are in German.
Re: Germany and smokeless propellant explosions?
Austria had a couple of smokeless/semi smokeless propellant manufacturing disasters very early on, which caused them to outlaw the production of nitrocellulose propellants for several decades, until the French adopted poudre B and small bore/high velocity infantry cartridges and forced everyone to play catch up (along with the rest of the world).
"The first nitrocellulose and smokeless propellants were made in Austria ca. 1857. In 1862, a guncotton factory in Austria was destroyed by an explosion, halting production for a time. This was followed by another devastating explosion in 1865 at a second guncotton facility, near Vienna Neustadt."
After this, they did manufacture a "less smokey" powder by adding nitrated sawdust to black powder during milling (worst of both worlds!), this stuff was not adopted by their military and was used mostly for shotgun shells/sporting purposes so far as I know.